NEW INNATE IMMUNITY MECHANISMSInflammation Types |
Feature |
Acute physiological |
Acute pathological |
Chronic pathological |
Onset |
Fast (minutes, hours) |
Fast (hours) |
Slowly (days) |
Duration |
Minutes, hours |
Hours, days |
Weeks, months, years |
Participation of cells |
Mainly neutrophils and macrophages |
Mainly neutrophils and macrophages |
Lymphocytes, monocytes, macrophages, eosinophils, mast cells, neutrophils |
Exudation |
No |
Yes |
No |
Thrombosis |
No |
Yes |
Maybe |
Tissue injury |
Uncommon and self-limited |
May be severe and progressive |
May be severe and progressive |
Connective tissue hyperplasia |
No |
No |
Yes |
Angiogenesis |
No |
No |
Yes |
Local and systemic signs |
Often subtle |
Prominent |
Prominent, less prominent or subtle |
Requires treatment |
No |
Yes |
Yes |
Life-thrteatening |
No |
Maybe |
Maybe |
Prerequisites |
Physiological activation of inflamasomes |
Pathological activation of inflammasomes (overactivation, activation deficiencies) |
|
|
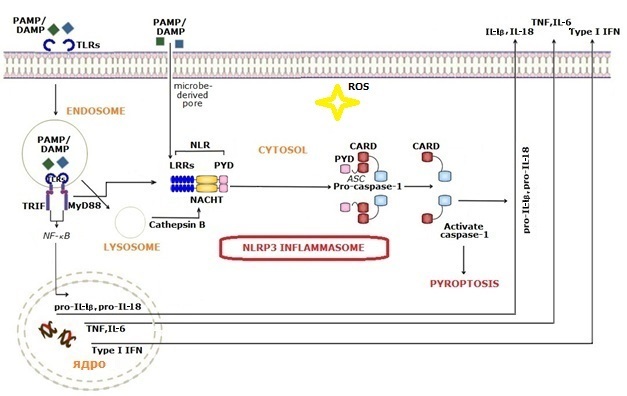
Inflammasome NLRP3 |
Inflammasome is a unit of innate immunity and inflammation that can be formed rapidly in response to infectious invaders and/or tissue injury. The inflammasome is a multiprotein complex expressed in myeloid cells, which activates caspase-1, processes pro-inflammatory cytokines IL-1β and IL-18, and induces cell pyroptosis, a process of programmed cell death different from apoptosis. The exact composition of an inflammasome depends on the activator, which initiates inflammasome assembly. Such activators are Microbe-Associated Molecular Patterns (PAMPs) and Damage-Associated Molecular Patterns (DAMPs) that act through a variety of Pattern Recognition Receptors (PRRs). Among the PRRs, TLRs can detect PAMPs/DAMPs/allergens in the extracellular environment and endosome, whereas NLRs and some other PRRs play a crucial role in sensing patterns in the intracellular compartments. |
|
The NLRP3 inflammasome is the best-studied inflammasome and seems to be activated by many microbes. Structurally,
NLRs contain LRR domains, a NACHT domain, and an effector domain, which can be either a pyrin domain (PYD), or
caspase recruitment domain (CARD), or another type. The effector pathways associated with the inflammasome act
at different levels.
Upon activation of the inflammasome, caspase-1 processes pro-IL-1β,
Mutation in NLRP3 gene (1q44), which encodes the protein cryopyrin, leads to Multisystem
Inflammatory Disease, a form of the congenital autoinflammatory disorder. Also, an excess
of pro-inflammatory cytokines may lead to "cytokine storm".
|
©V.V.Klimov