|
|

PATTERNS AND PATTERN RECOGNITION RECEPTORS (PRR)
Molecular Patterns
Designation
Name
Features
Functions
Immunopathology
PAMP
Pathogen Associated Molecular Patterns
Small microbe-derived molecules, which contain a conserved motif (bacterial flagellin, peptidoglycan, lipopolysaccharide (endotoxin, LPS), viral dsRNA and ssRNA, and unmethylated CpG motifs of DNA)
They may initiate different reactions of innate immunity and even trigger adaptive responses
Immunodeficiences, autoinflammatory disorders, toxic shock syndrome
AAMP
Allergen Associated Molecular Patterns
Oligomeric components of allergenic molecules
They facilitate cross-linking of IgE with allergen
IgE-dependent allergic disorders
DAMP
Damage Associated Molecular Patterns
Small substances released outside the damaged tissues and cells (heat-shock proteins, extracellular matrix's (ECM's) proteins, S100, hyaluronan fragments, and non-protein substances such as DNA, ATP, uric acid, and heparin)
They provide the body with danger signals
Autoinflammatory disorders, toxic shock syndrome
TAMP
Tumor Associated Molecular Patterns
Small conserved components from tumor cells
They may like "double-edged sword" upregulate both anti-tumor defense and, conversely, tumor growth and metastasis
Tumor progression and metastasis
Pattern Recognition Receptors (PRR)
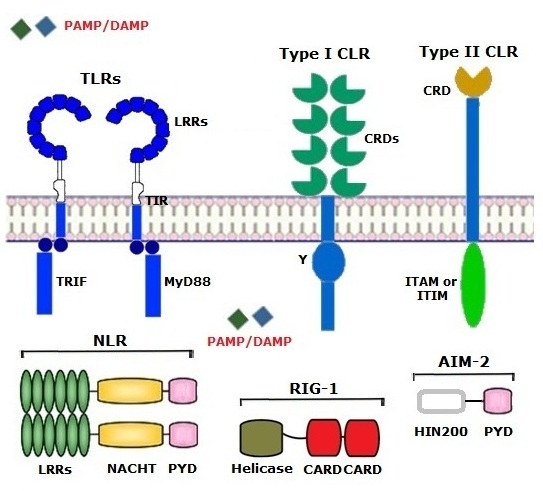
The term "NACHT" comes from NAIP - an apoptosis inhibitor protein. C2TA - a Class II HLA transcription activator, HET-E – an incompatibility locus protein from Podospora anserina, and TP1 – a telomerase-associated protein. A NACHT domain consists of 7 different conserved motifs, contains 300–400 amino acids, and exerts NTPase activity. A PYD domain takes part in the formation of an inflammasome, responsible for activating caspase-1, pro-inflammatory cytokines such as IL-1β and IL-18, pyroptosis, and starting the inflammatory process.
RIG-1-Like Receptors (RLR) are present in the cytoplasm and consist of an RNA helicase domain and CARDs (Caspase Activation and Recruitment Domains). A ligand for RLRs is a viral PAMP, dsRNA. Activation of RLRs leads to type I interferon production.
AIM-2-Like Receptors (ALR) are located in the cytoplasm and nucleus and contain a certain number of HIN200 domains and PYD domain. The term "HIN200" comes from "Hematopoietic expression, Interferon-inducible nature, and Nuclear localization of 200 amino acids." A ligand for ALRs is dsDNA of bacteria or viruses. Activation of AIM-2 results in the formation of AIM-2 inflammasome, production of pro-inflammatory cytokines and type I interferons.©V.V.Klimov
Patterns, are low-molecular substances evoking reactions of innate immunity and adaptive responses with no memory. Patterns are ligands for pattern recognition receptors (PRR).
Toll-Like Receptors (TLR) are expressed on the (i) membranes of macrophages, neutrophils, dendritic cells, lymphocytes, epitheliocytes, platelets, splenocytes, atherosclerotic plaques, etc., and present in the (ii) endosomes of the cells. The term "toll" comes from drosophila, in which TLRs were first identified. TLRs contain leucine-rich repeat (LRR) domains and a Toll/Interleukin1 Receptor (TIR) domain, which is linked to signaling molecules including MyD88, TRIF, etc. After binding to ligands, most TLRs transduce signals in MyD88-dependent and TRIF-dependent pathways and eventually lead to the activation of inflammasome and pyroptosis, a base of the physiological inflammation. TLRs can recognize both PAMPs and DAMPs, as well as allergens, and provide a link between innate and adaptive immunity since they can interact with antigens too. A pathogen bound to TLRs may be engulfed, digested, and presented to lymphocytes in antigenic form.
C-type Lectin Receptors (CLR) are categorized by the structure in type I and type II, and by recognition motif in groups I and II. They all are bound to the cell membranes and mainly involved in fungal recognition and endocytosis. Analogous to TLR, CLRs may recognize both "patterns" and antigens being a bridge between innate and adaptive immunity. Type I CLRs consist of several Carbohydrate Recognition Domains (CRDs) linked to cytoplasmic tails with a Y motif for signaling. Type II CLRs contain only one CRD, and their cytoplasmic tail includes either ITIM (immunoreceptor tyrosine-based inhibitory motif), or ITAM (immunoreceptor tyrosine-based activating motif), or other sequence motifs to be a start point of signaling pathways. The members of type II CLRs are predominant in comparison with type I CLRs and are at the cutting edge of current research. They play many roles in anti-fungal, anti-mycobacterial, anti-tumor immunity, allergy, and maintenance of tissue homeostasis. For example, the study of Dectin-1 has revolutionized our understanding of human body-fungal interactions, whereas research on Dectin-2 has discovered that some CLRs may sense allergens such as house dust mite allergens as well as Allergen-Associated Molecular Patterns (AAMP).
Soluble type CLRs are related to "acute phase" proteins.
NOD-Like Receptors (NLR) are localized in the cytosol. The term "NOD" comes from a Nucleotide-binding Oligomerization Domain, which binds nucleotide triphosphate. NOD1 receptors recognize peptidoglycan only of Gram-negative bacteria, whereas NOD2 molecules recognize intracellular muramyl dipeptide, peptidoglycan of both Gram-positive and Gram-negative bacteria. NLRs commonly consist of LRR domains, a central NACHT domain and an effector domain, which may be one of five types, including PYD domain (Pyrin Domain).