|

REGULATION OF IMMUNE RESPONSES
Immune Tolerance
|
 |
Sir F.M. Burnet (Australia) and Sir P.B. Medawar (UK) won the 1960 Nobel Prize for their research on
immune tolerance.
|
Immune tolerance, the antipode of an adaptive immune response, is a state of the
immune system when there is unresponsiveness to a specific antigen, which must be called tolerogen.
Tolerance is commonly accepted to be an active process both at the central and peripheral levels and with both
engagements of tolerogenic T cells and B cells. The tolerant state is not absolute and rarely complete.
There may be T-cell tolerance, more often low-dose dependent and long-term, and B-cell
tolerance, more frequently high-dose dependent and short-term.
|
|
Under natural conditions, the immune system is tolerant to:
(1) self-antigens (or autoantigens of the body);
(2) antigens of own symbiotic and opportunistic microbes in the steady-state;
(3) food proteins;
(4) allergens of the environment, and
(5) antigens of spermatozoa (inside women) and the father's antigens of the fetus (in pregnant women).
|
|
There are several physiological processes for the maintenance of natural tolerance:
(1) central mechanisms (T-cell and B-cell clonal deletion);
(2) peripheral mechanisms:
– clonal anergy;
– activation-induced apoptosis;
– clonal ignorance;
- natural tolerance maintenance system activity.
The system of natural tolerance maintenance includes the functioning of the following components:
|
|
(1) tolerogenic dendritic cells (tDC);
(2) peripheral regulatory T (pTreg) cells and their subsets;
(3) regulatory B (Breg) cells;
(4) macrophages (M2);
(5) myeloid-derived suppressor cells (MDSC);
(6) pro-tolerogenic (immunosuppressive) cytokines IL-10, TGF-β, IL-27, IL-35;
(7) coinhibitory molecules CTLA-4, PD-1, BTLA, LAG-3, etc.;
(8) pro-tolerogenic neurotransmitters and neuropeptides;
(9) tolerogenic microbiota.
|
|
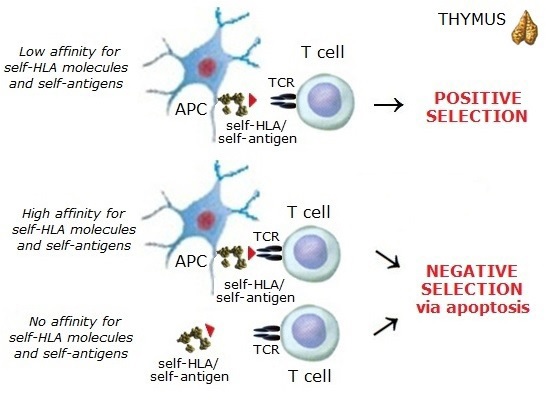
Central tolerance of T cells occurs during thymic development with the bulk of
self-reactive T cells eliminated during negative selection in the thymus termed "clonal deletion."
However, the thymic selection is not a perfect process, and hence a part of self-reactive
T cells may frequently be released into the periphery.
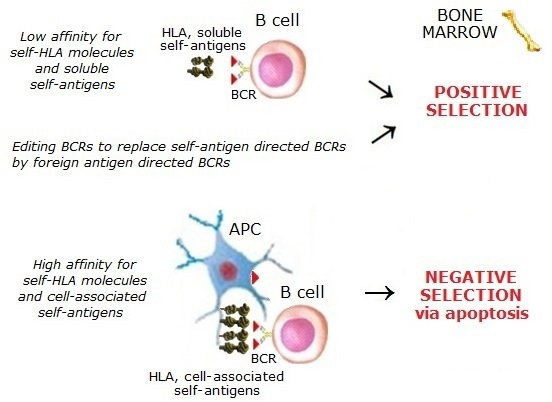
Central B-cell tolerance is regulated by clonal deletion of immature B cells in the
bone marrow, engaging apoptosis and receptor editing. However, survived self-reactive low-affinity B cells enter
the bloodstream. Some self-reactive B-cells in the bone marrow get further chances to express alternative BCRs
through a gene rearrangement process known as receptor editing rather than undergo apoptosis.
|
 |
 |
|
Peripheral tolerance is provided with a variety of different mechanisms.
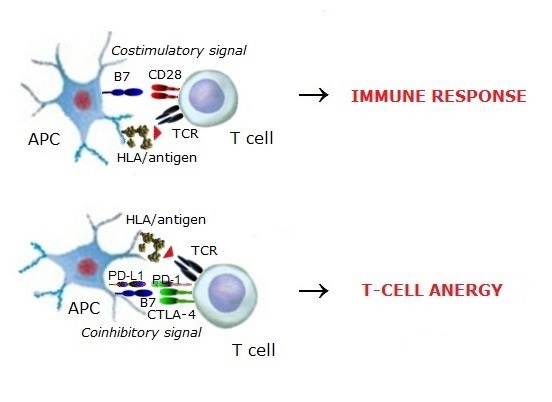
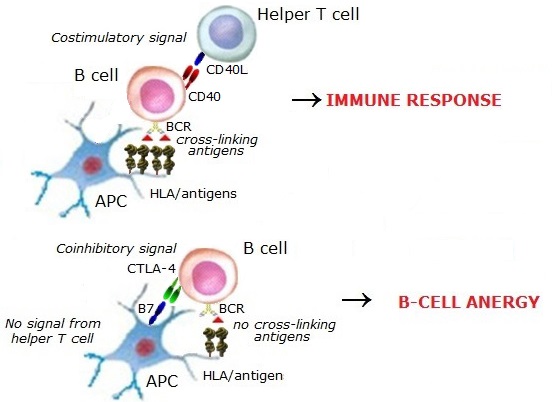
1. Clonal anergy is a silent state of self-reactive B cells and T cells in the periphery.
After negative thymic selection, part of survived self-reactive low-affinity T cells occur in the periphery. Another
part, the high-affinity self-reactive T cells whose TCRs are directed to self-antigens not expressed within
the thymus, also occur in the periphery. Peripheral self-reactive T cells become anergic due to losing the valuable
TCR signaling and getting coinhibitory signals from the expressed coinhibitory molecules like CTLA-4 and PD-1.
The antigen-recognizing receptors of B cells at the immature stages are directed to
soluble self-antigens at a low concentration. These B cells escaped from the negative selection in the bone marrow
and appeared in the periphery. When self-reactive B cells become anergic, they lose the BCR signaling, get
coinhibitory signals from the coinhibitory molecules, and do not receive aid from helper T cells.
|
|
 |
 |
|
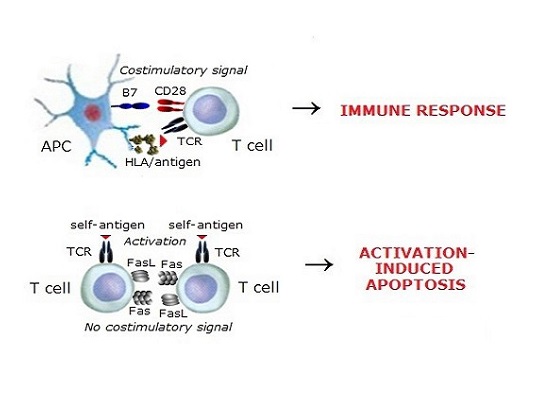
2. Activation-induced apoptosis is the apoptosis in self-reactive T cells previously
activated through TCR. TCR ligation upregulates CD95 expression and induces the expression of CD95L (CD178).
Apoptosis is mediated by the activation of the Fas (CD95) pathway, which is promoted upon the ligation of cell
surface CD95 by CD95L (FasL) and can occur by the interaction of CD95 and CD95L on the same self-reactive T cell.
However, costimulation of T cells by the direct ligation of CD28 inhibits activation-induced apoptosis in these cells.
Thus, for activation-induced apoptosis in previously activated self-reactive T cells, maintenance of the natural
tolerance and prevention of autoimmune disorders in target organs, the absence of a costimulatory signal is necessary.
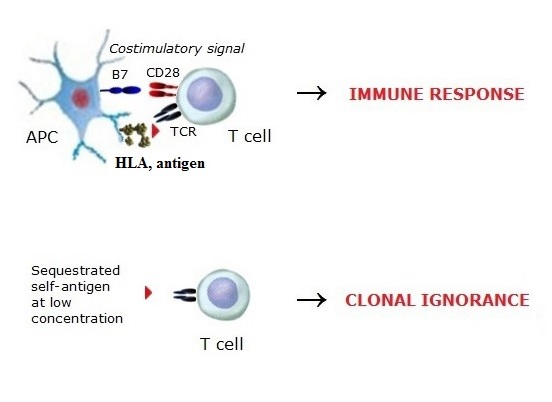
3. Clonal ignorance is the unresponsiveness of T cells in the periphery to
self-antigens at a low concentration. However, self-ignorant T cells represent one of the most significant threats
to the maintenance of tolerance. There are immune-privileged organs such as some parts
of the brain, the anterior chamber of the eye, the testes, etc. Physiological barriers separate the blood from these
self-antigens (so-called "sequestrated or cryptic determinants"), but upon certain conditions (e.g., trauma)
self-antigens can enter the bloodstream at a high concentration and lead to a tolerance breakdown.
|
|
 |
 |
|
Pathologic tolerance occurs under conditions that pathologically suppress
the immune responses to antigens (e.g., in tumor growth and chronic infection). Immune tolerance may be
artificial tolerance achieved for medical purposes (e.g., in transplantation
and atopic allergies). Allergen-specific immunotherapy (AIT) enables the formation of allergen tolerance for a long time.
|
©V.V.Klimov
|
|







