|
|

CELLS OF THE IMMUNE SYSTEM
Monocytes and Macrophages
Monocytes make up 4-8 % of bloodstream leukocytes. Monocytes originate from the bone marrow and are divided into three groups: 1) classical (80-90%), 2) non-classical "patrolling" (5-10%) è 3) intermediate (5-10%) cells.
In the steady-state, classical monocytes can circulate for about 24 h, then engraft into peripheral monocyte reservoirs, or differentiate into non-classical patrolling monocytes or tissue-resident macrophages. Non-classical "patrolling" monocytes circulate for about 7 days. All monocytes are pro-inflammatory cells, which infiltrate the inflammatory sites and promote inflammatory processes. Monocytes are a source for the development of resident macrophages, inflammatory dendritic cells, and Langerhans cells. However, there are macrophages of non-monocyte origin.
Macrophages discovered by 1908 Nobel Laureate E. Mechnikof play an essential role in both innate and adaptive immunity. They can phagocyte microbes, immune complexes, and apoptotic bodies or extracellular vesicles in a process known as efferocytosis,engulf, process, and present antigens to lymphocytes as well as secrete enormous bioactive substances: cytokines, chemokines, complement proteins, enzymes including metalloproteinases, arachidonic acid metabolites, nitric oxide, ROS, etc.
There are two functional and phenotypic macrophage populations:
involved in the Th1 polarization via IL-12 secretion, CD4+T-mediated responses, immune inflammation, and production of inflammatory cytokines: IL-1β, IL-6, and TNF-α (responsible for cytokine storm) , and
inflammatory (Ì1),
anti-inflammatory (Ì2), engaged in immune tolerance maintenance and tissue regeneration. However, Ì2 macrophages can upregulate tumor growth.Type 1 macrophages (M1),
CD64+, originate from monocytes and function as participants of antigen processing, antigen presentation for CD4+T cell-mediated immune response, and any immune inflammation, including type IV hypersensitivity. The excitement of M1 macrophages depends on many factors, among which IFN-γ is a crucial activator for M1 polarization.
Type 2 macrophages (M2),
CD163+CD206+, are polarized by IL-4, IL-10, and IL-13. They are more heterogenous cells, in contrast to type I macrophages, and subdivided into four subsets:
Ì2à cells (alternatively activated macrophages) exert potent immunosuppressive properties including inflammation downregulation, tissue repair, wound healing, and pattern-associated molecular patterns (PAMP) recognition;
Ì2b cells (type II activated macrophages) are ambivalent;
M2c cells (deactivated macrophages) fulfill the clearance of dead cells via phagocytosis;
M2d cells (tumor-associated macrophages) (TAM) promote the growth of cancerous cells.
The extracellular vesicles undergoing efferocytosis by M2 macrophages contain potent bioactive substances required for the interaction between dying cells and macrophages, a phenotypic shift within macrophages towards M2 phenotype, and prevention of tissue damage.
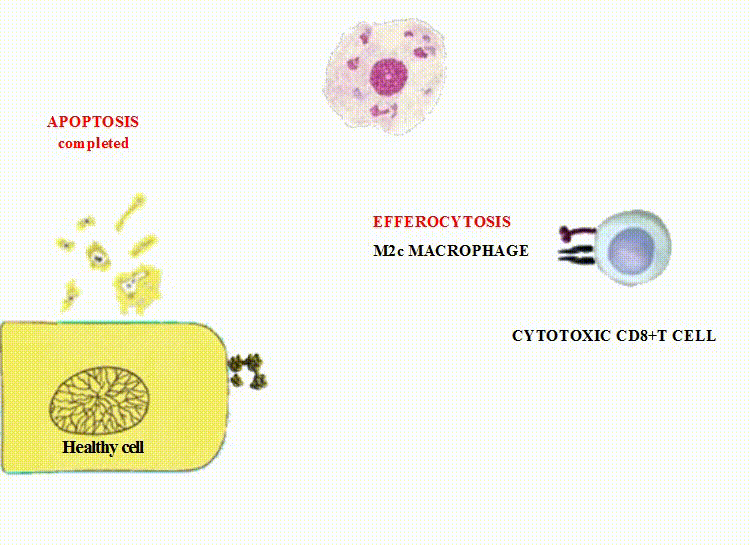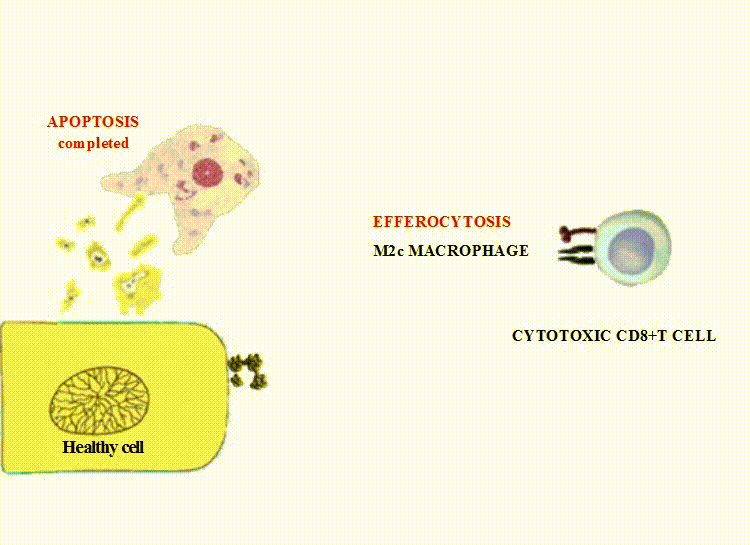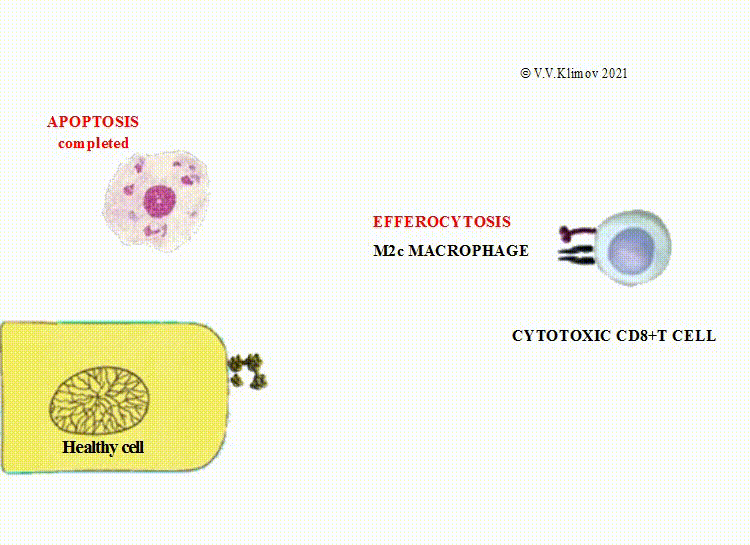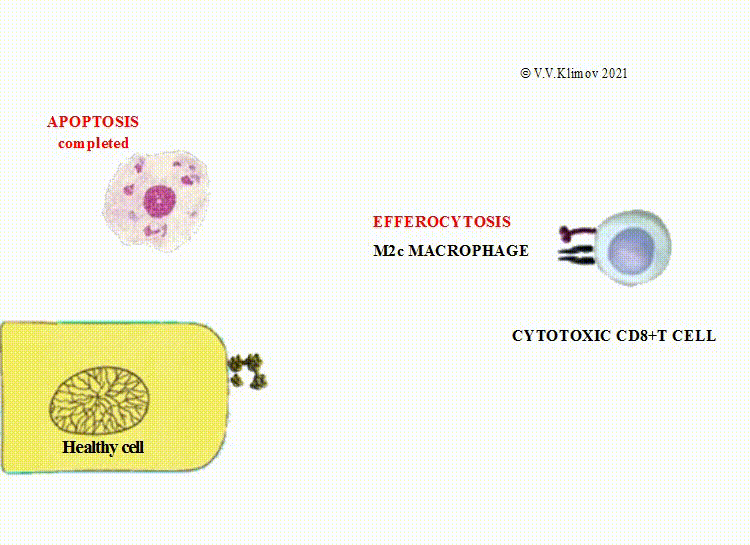
Resident macrophages are the following:
- Kupffer's cells (the liver)
- Mesangial cells (the kidney)
- Peritoneal macrophages (the peritoneum)
- Pleural macrophages (the pleura)
- Pulmonary alveolar & interstitial macrophages (the lung)
- Microglial cells (the central nervous system)
- Macrophages of the bone marrow
- Macrophages of spleen's marginal zone and red pulp
- Macrophages of the lymph nodes
- Osteoclasts (the bone)
- Hofbauer's cells (the genitourinary tract)
©V.V.Klimov