
COMPLEMENTComplement Activation via Classical Pathway |
|
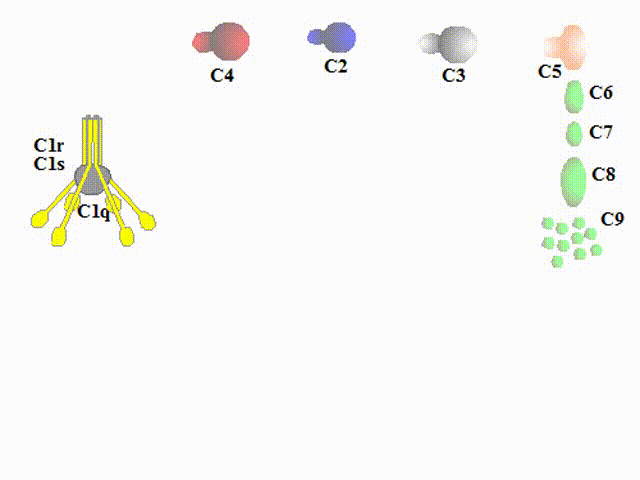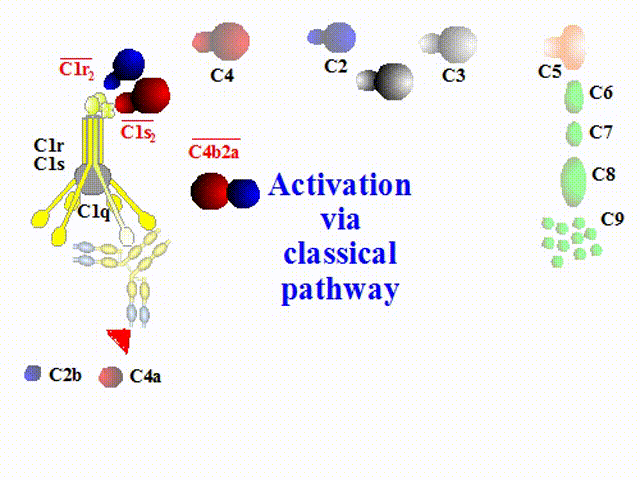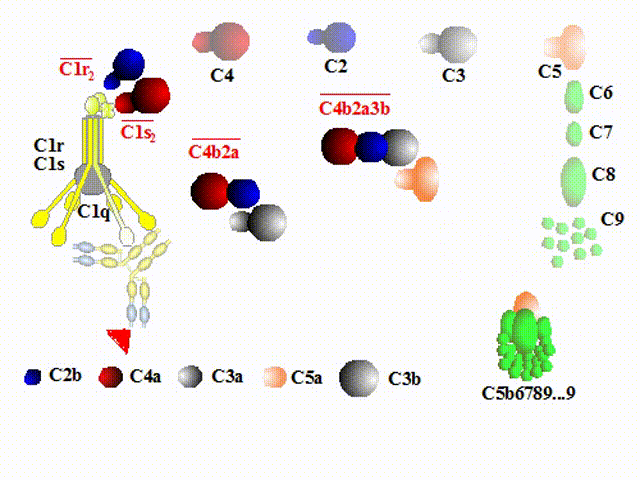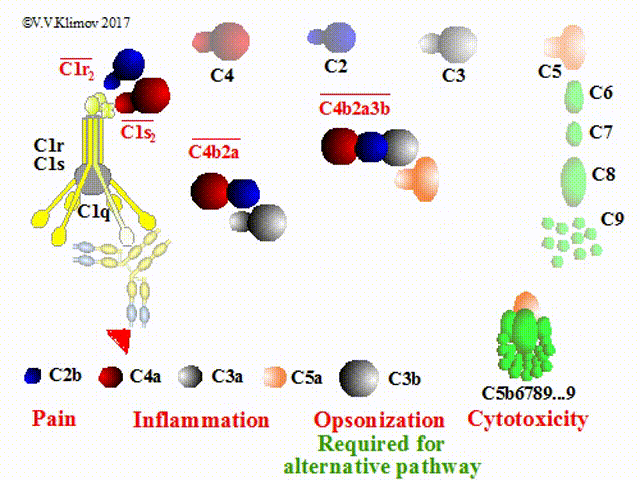
The classical pathway is activated by immune complexes. Conformational change in
an immunoglobulin molecule bound to an antigen leads to disclosure of the site for binding C1. The C1 consists of
6 subcomponents C1q of a tulip-like structure including a globular head for attaching an antibody, two C1r and
two C1s. When the antibody attached to a particle of interest (e.g., bacterial cell surface or viral envelope) binds
to C1q, C1r generates an active enzyme, C1s esterase. Substrates for the enzyme C1s are C2 and C4 that are
cleaved into some fragments, C2a/C2b, and C4a/C4b respectively. C4b and C2b constitute a new enzyme, C4b2b,
known as the classical pathway C3 convertase, which acts on C3.
As a rule, during following events a smaller fragment (-a) will exert powerful biological qualities outside
the cascade, whereas a larger fragment (-b) will participate in the formation of new molecules of the complement
cascade.
©V.V.Klimov |